The Adsorption of Iron (Fe) in Dyeing and Washing Waste of Jumputan Fabric Using Active Carbon from Tea Grounds
Penyerapan Logam Besi (Fe) Dalam Limbah Pewarnaan Dan Pencucian Industri Kain Jumputan Menggunakan Karbon Aktif Dari Ampas Teh
DOI:
https://doi.org/10.21070/pels.v2i2.1226Keywords:
Activated Carbon, Adsorption, Tea Grounds, Jumputan Weaving IndustryAbstract
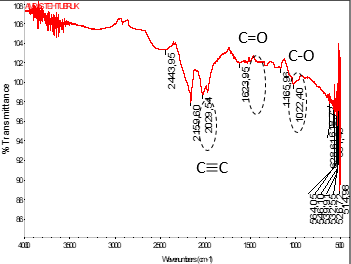
The iron (Fe) contained in the waste dyeing process and the washing process of the jumputan fabric weaving industry is relatively high; this can cause water pollution in the waters around the jumputan fabric weaving industry if the iron content is not adsorbed. One of the methods used to decrease iron content (FE) is adsorption. The adsorbent used for this iron adsorption (Fe) process is activated carbon made from black tea grounds. Before adsorbing iron, making activated carbon from black tea grounds is carried out first, namely by carbonating the tea grounds at the temperature of 500oC for 20 minutes, then using a 4% NaOH solution for 24 hours. The quality results of activated carbon from black tea grounds produced are 19,1635% of volatile matter, 9,1465% of water content, 2,0911% of ash content, 69,5989% of pure activated carbon, and 0.2664 mg/L adsorptions of methyl blue, and these characteristics meet the quality standard of SNI no.06-3730-1995. Activated carbon that is produced and has filled the quality standards is then used to absorb iron (Fe) content in the dyeing and washing process waste of the jumputan weaving industry. The adsorption carried out the process for 72 hours with adsorbent mass variables of 1 g, 2 g, 4 g, 8 g, and 10 g. Then the levels of iron (Fe) were analyzed using AAS. The best condition for adsorption of iron content in the waste dyeing process of jumputan fabric was obtained at a mass of 10 g of adsorbent was 32.437 mg/L with an adsorption efficiency of 99.68%, the same condition for adsorption of iron content in washing waste that was equal to 9.998 mg/L with an adsorption efficiency of 99.68%.
Downloads
References
H. P. Asmaningrum and Y. P. Pasaribu, “PENENTUAN KADAR BESI (Fe) DAN KESADAHAN PADA AIR MINUM ISI ULANG DI DISTRIK MERAUKE,” Magistra: Jurnal Keguruan dan Ilmu Pendidikan, vol. 3, no. 2, pp. 95–104, Jul. 2016, doi: 10.35724/magistra.v3i2.592. [Online]. Available: https://ejournal.unmus.ac.id/index.php/magistra/article/view/592. [Accessed: Aug. 09, 2022].
R. G. Muhajjalin, I. Agawijaya, B. Santoso, and J. Suryadi, “Perbandingan Efektivitas Ampas Teh Hitam dan Ampas Teh Hijau sebagai Adsorben Ion Logam Cr (VI),” Fullerene Journ. of Chem, vol. 6, no. 2, p. 9, 2021, doi: doi 10.37033/fjc.v6i2.327.
R. F. Azzahra and M. Taufik, “BIO-ADSORBEN BERBAHAN DASAR LIMBAH AMPAS TEH (CAMELLIA SINENSIS) SEBAGAI AGENT PENYERAP LOGAM BERAT FE DAN PB PADA AIR SUNGAI,” KINETIKA, vol. 11, no. 1, pp. 65–70, 2020 [Online]. Available: https://jurnal.polsri.ac.id/index.php/kimia/article/view/3113. [Accessed: Aug. 09, 2022].
L. Purwaningsih, R. Rachmaniyah, and P. Hermiyanti, “PENURUNAN KADAR BESI (II) PADA AIR BERSIH MENGGUNAKAN AMPAS DAUN TEH DIAKTIVASI,” KESLING, vol. 17, no. 2, Jul. 2019, doi: 10.36568/kesling.v17i2.1124. [Online]. Available: http://journal.poltekkesdepkes-sby.ac.id/index.php/KESLING/article/view/1124. [Accessed: Aug. 09, 2022].
I. S. Hardyanti, I. Nurani, D. S. Hardjono HP, E. Apriliani, and E. A. P. Wibowo, “Pemanfaatan Silika (SiO2) dan Bentonit sebagai Adsorben Logam Berat Fe pada Limbah Batik,” j. St. terap, vol. 3, no. 2, Oct. 2017, doi: 10.32487/jst.v3i2.257. [Online]. Available: http://jurnal.poltekba.ac.id/index.php/jst/article/view/257. [Accessed: Aug. 09, 2022].
I. Iswadi, “Pemanfaatan Karbon Aktif Dari Ampas Teh Sebagai Adsorben Dalam Pengolahan Methyl Blue,” Institut Muhammadiyah Palembang, 2013.
I. K. Wijaya, Y. Farra Yulia, and K. Udyani, “PEMANFAATAN DAUN TEH SEBAGAI BIOSORBEN LOGAM BERAT DALAM AIR LIMBAH (REVIEW),” envirotek, vol. 12, no. 2, pp. 25–33, Nov. 2020, doi: 10.33005/envirotek.v12i2.55. [Online]. Available: http://envirotek.upnjatim.ac.id/index.php/envirotek/article/view/55. [Accessed: Aug. 09, 2022].
D. Suwazan and N. Nurhidayanti, “Efektivitas Kombinasi Kitosan dan Ampas Teh Sebagai Adsorben Alami dalam Menurunkan Konsentrasi Timbal Pada Limbah Cair PT PXI,” J. Ilmu Lingk., vol. 20, no. 1, pp. 37–44, Jan. 2022, doi: 10.14710/jil.20.1.37-44. [Online]. Available: https://ejournal.undip.ac.id/index.php/ilmulingkungan/article/view/41989. [Accessed: Aug. 09, 2022].
D. A. Pratama, “EFEKTIVITAS AMPAS TEH SEBAGAI ADSORBEN ALTERNATIF LOGAM Fe DAN Cu PADA AIR SUNGAI MAHAKAM,” JIP UNTIRTA, vol. 6, no. 3, Jun. 2017, doi: 10.36055/jip.v6i3.1560. [Online]. Available: http://jurnal.untirta.ac.id/index.php/jip/article/view/1560. [Accessed: Aug. 09, 2022].
V. Viena, B. Bahagia, and Z. Afrizal, “Produksi Karbon Aktif dari Cangkang Sawit dan Aplikasinya Pada Penyerapan Zat Besi, Mangan Dan ph Air Sumur,” JSE, vol. 5, no. 1, Dec. 2019, doi: 10.32672/jse.v5i1.1660. [Online]. Available: http://ojs.serambimekkah.ac.id/index.php/jse/article/view/875%20-%20882. [Accessed: Aug. 09, 2022].